Renewable polyesters derived from 10-undecenoic acid and vanillic acid with versatile properties†
Abstract
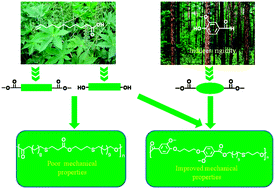
Vanillic acid and 10-undecenoic acid, based on lignin and castor oil, respectively, were used as raw materials for the synthesis of thermoplastic polyesters (PEs). Thiol–ene click chemistry was used to produce renewable aliphatic diols and diester from 10-undecen-1-ol and methyl 10-undecenoate, respectively. Two renewable aromatic diesters were prepared from methyl vanillate. A series of thermoplastic PEs with tunable thermo-mechanical properties were synthesized by polycondensation of the diols and the diesters. These PEs were characterized using FTIR, 1H-NMR, and SEC, and their thermo-mechanical properties were studied using thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and dynamic mechanical analysis (DMA). The weight-average molecular weight (Mw) of the PEs was found to be in the range of 26 700–57 200 g mol−1. The bio-based PEs exhibited semi-crystalline to amorphous behavior with their glass transition temperature (Tg) values ranging from −12.7 to 13.0 °C. The incorporation of the aromatic segments into the PE backbone was found to be an outstanding feature, improving the mechanical properties. The Young's modulus and elongation at break of the aromatic PEs were found to be in the range of 50.0–99.7 MPa and 12.7–43.7%, respectively.

 Please wait while we load your content...
Please wait while we load your content...