Radical emulsion polymerization with chain transfer monomer: an approach to branched vinyl polymers with high molecular weight and relatively narrow polydispersity†
Abstract
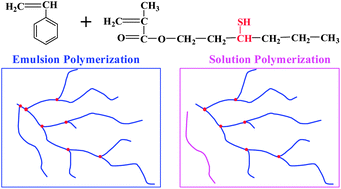
Radical polymerization with 3-mercapto hexyl methacrylate as the chain transfer monomer (CTM) to prepare branched vinyl polymers with high molecular weights and relatively narrow polydispersities has been carried out in aqueous emulsion. Potassium persulfate was used as the initiator, and sodium dodecyl benzene sulfate or hexadecyl trimethyl ammonium bromide was used as the emulsifier. The obtained polymers were characterized using NMR and size exclusion chromatography. Compared with polymers obtained from solution polymerization and results reported in the literature, branched polymers can be prepared at higher monomer/branching monomer feed ratios (100/25) with high monomer conversion (usually >95%) without gelation. The obtained branched polymers also showed considerably higher molecular weights and relatively narrower polydispersities at the same feed ratio of monomer/branching monomer. The unique radical termination mechanism in emulsion reaction determines that soluble branched polymers with high molecular weight and relatively narrow polydispersity can be prepared at a wide range of monomer/CTM feed ratios without gelation.

 Please wait while we load your content...
Please wait while we load your content...