A comparative study of polymers containing naphthodifuranone and benzodifuranone units in the main chain†
Abstract
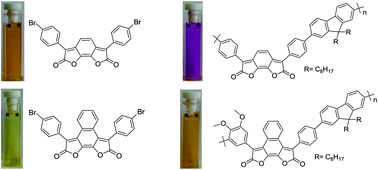
The synthesis of two new polymers containing the naphtho[2,1-b:3,4-b′]difuran-2,9-dione (1,10-NDF) or the ortho-benzo[1,2-b:6,5-b′]difuran-2,7-dione (1,8-BDF) chromophore in the main chain is described. The polymers P1 and P2′ were prepared upon Suzuki coupling of either 3-(4-bromophenyl)-8-(3-bromo-4,5-dimethoxyphenyl)-naphtho[2,1-b:3,4-b′]difuran-2,9-dione (M1) or 3,6-bis(4-bromophenyl)-1,2,3,5,6,7-hexahydrobenzo[1,2-b:6,5-b′]difuran-2,7-dione (M2′) and 2,2-(9,9-dioctyl-9H-fluorene-2,7-diyl-)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (M3), respectively. P2′ was subsequently converted into the oxidized form P2 upon reaction with nitrobenzene. Optical and electrochemical properties of P1 and P2 are described and compared with the properties of previously prepared polymer P3, which was synthesized upon Suzuki coupling of symmetrical 3,8-bis(4-bromophenyl)naphtho[2,1-b:3,4-b′]difuran-2,9-dione (M4) and M3. While the NDF-based polymers P1 and P3 exhibit absorption maxima around 480 nm, the BDF-based polymer P2 shows a maximum at 555 nm. Quantum chemical studies by the density functional theory method show that the BDF-based polymer is more planar than the polymers based on NDF. The excitation of the polymers causes a strong electron transfer from the backbone to the NDF/BDF core units. 1,10-NDF-based polymers exhibit excellent photostability in UV-light, while the 1,8-BDF-based polymer is less stable, if irradiated with a 200 W Hg lamp. All polymers exhibit low band gaps of 1.31 to 1.66 eV.

 Please wait while we load your content...
Please wait while we load your content...