A biomimic pH-sensitive polymeric prodrug based on polycarbonate for intracellular drug delivery†
Abstract
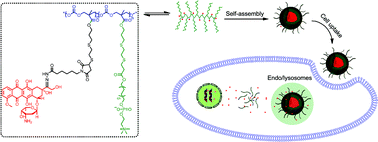
A biodegradable and endosomal pH-sensitive polymeric prodrug poly(5-methyl-5-allyloxycarbonyl-1,3-dioxan-2-one)-graft-12-acryloyloxy dodecyl phosphorylcholine-co-6-maleimidocaproyl-doxorubicin (PMAC-graft-(ADPC-co-Mal-DOX) was synthesized by ring-opening polymerization (ROP) and “click” reaction. DOX was conjugated to the polymer by hydrazone bonds which would result in a pH-sensitive controlled release of drug. The polymeric prodrug can form a self-assembled micellar structure which was confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Flow cytometry and fluorescence microscopy results demonstrated that prodrug micelles could be internalized by cancer cells remarkably. In vitro drug release studies showed that the release of DOX was faster at endosomal pH (pH = 5.0) than at normal physiological environment (pH = 7.4). Moreover, this prodrug exhibited high cytotoxicity against HepG2 cells and HeLa cells, indicating its great potential for cancer therapy.

 Please wait while we load your content...
Please wait while we load your content...