Thymine photodimer formation in DNA hairpins. Unusual conformations favor (6 − 4) vs. (2 + 2) adducts†‡
Abstract
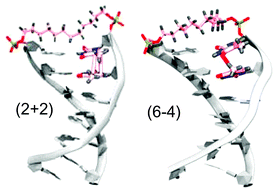
The photochemical reactions of eleven synthetic DNA hairpins possessing a single TT step either in a base-paired stem or in a hexanucleotide linker have been investigated. The major reaction products have been identified as the cis–syn (2 + 2) adduct and the (6 − 4) adduct on the basis of their spectroscopic properties including 1D and 2D NMR spectra, UV spectra and stability or instability to photochemical cleavage. Product quantum yields and ratios determined by HPLC analysis allow the behaviour of the eleven hairpins to be placed into three groups: Group I in which the (2 + 2) adduct is the major product, as is usually the case for DNA, Group II in which comparable amounts of (2 + 2) and (6 − 4) adducts are formed, and Group III in which the major product is the (6 − 4) adduct. The latter behaviour is without precedent in natural or synthetic DNA and appears to be related to the highly fluxional structures of the hairpin reactants. Molecular dynamics simulation of ground state conformations provides quantum yields and product ratios calculated using a single parameter model that are in reasonable agreement with most of the experimental results. Factors which may influence the observed product ratios are discussed.
- This article is part of the themed collection: Dedicated to the memory of Prof. Nicholas J. Turro

 Please wait while we load your content...
Please wait while we load your content...