Synthesis of thyminyl stilbazoles and their photo-reactivity†
Abstract
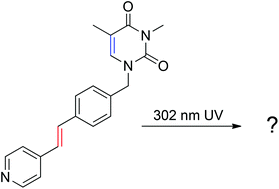
Photo-reactions of molecules with two [2π + 2π]-cycloaddition sites in the solid state are reported. Four thyminyl stilbazoles having both a stilbazole olefin and a thyminyl olefin were synthesized using the Heck reaction of halo-pyridine substrates with vinylbenzyl thymine or methylated vinylbenzylthymine. Only one of them, methylated vinylbenzylthymine with 4-pyridine, was photoreactive and formed a head-to-tail stilbazole dimer. The crystal structures of thyminyl stilbazoles and the stilbazole dimer were used to investigate the structure–reactivity relationships.

 Please wait while we load your content...
Please wait while we load your content...