Synthesis of full length and truncated microcin B17 analogues as DNA gyrase poisons†
Abstract
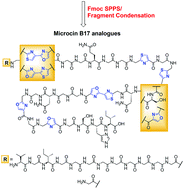
Microcin B17 (MccB17) is a post-translationally modified peptide containing thiazole and oxazole heterocycles that interrupt the peptide backbone. MccB17 is capable of poisoning DNA gyrase through stabilization of the gyrase-DNA cleavage complex and has therefore attracted significant attention. Using a combination of Fmoc-strategy solid-phase peptide synthesis and solution-phase fragment assembly we have prepared a library of full-length and truncated MccB17 analogues to investigate key structural requirements for gyrase-poisoning activity. Synthetic peptides lacking the glycine-rich N-terminal portion of the full-length sequence showed strong stabilization of the gyrase-DNA cleavage complex with increased potency relative to the full-length sequences. This truncation, however, led to a decrease in antibacterial activity of these analogues relative to their full-length counterparts indicating a potential role of the N-terminal region of the natural product for cellular uptake.

 Please wait while we load your content...
Please wait while we load your content...