Stereoselective tandem synthesis of thiazolo fused naphthyridines and thienopyridines from o-alkynylaldehydes via Au(iii)-catalyzed regioselective 6-endo-dig ring closure†
Abstract
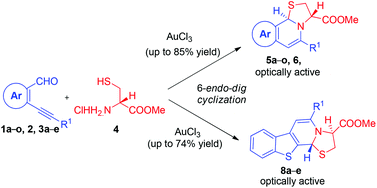
An operationally simple approach for the stereoselective tandem synthesis of novel thiazolo fused naphthyridines 5a–o and thienopyridines 8a–e by the reaction of o-alkynylaldehydes with L-cystine methyl ester hydrochloride via Au(III)-catalyzed regioselective 6-endo-dig ring closure under mild reaction conditions is described. It is noteworthy that alkynes bearing an alkyl and a strong electron-withdrawing nitro group successfully afforded the desired products in good yields.

 Please wait while we load your content...
Please wait while we load your content...