Copper-catalysed intramolecular O-arylation: a simple and efficient method for benzoxazole synthesis†
Abstract
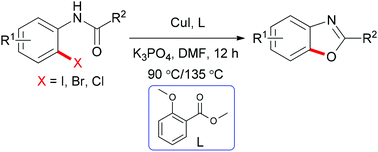
A wide range of 2-substituted benzoxazoles can be efficiently synthesized from N-(2-iodo-/bromo-phenyl)benzamides, and even the less reactive N-(2-chlorophenyl)benzamides, via Cu-catalysed intramolecular coupling cyclization reactions using methyl 2-methoxybenzoate as the ligand under mild reaction conditions. In addition, the benzoxazoles can be easily prepared from the primary amides coupling with o-dihalobenzenes in a single step.

 Please wait while we load your content...
Please wait while we load your content...