Thermodynamic epimeric equilibration and crystallisation-induced dynamic resolution of lobelanine, norlobelanine and related analogues†
Abstract
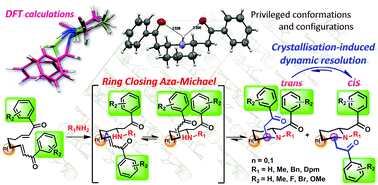
The step-economical synthesis of lobelanine involving a ring closing double aza-Michael (RCDAM) reaction is revisited and successfully extended to the synthesis of various configurationally more stable analogues. Owing to the presence of a configurationally labile β-aminoketone subunit, lobelanine is prone to self-catalyze mutarotation in solution. Through the synthesis of original lobelanine analogues, we studied the influence of (i) the size of the central heterocycle, (ii) the bulkiness of the nitrogen protecting group, and (iii) the phenacyl arm substituent on the thermodynamic equilibrium and its displacement by crystallisation-induced dynamic resolution (CIDR). We demonstrated that fine structural tuning combined with optimized CIDR conditions favours the first efficient diastereoselective synthesis of lobelanine's analogues.

 Please wait while we load your content...
Please wait while we load your content...