Lewis acid promoted construction of chromen-4-one and isoflavone scaffolds via regio- and chemoselective domino Friedel–Crafts acylation/Allan–Robinson reaction†‡
Abstract
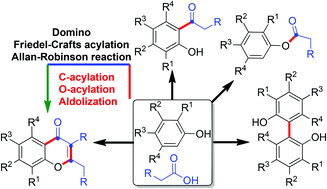
A facile and efficient synthesis of chromen-4-one and isoflavone frameworks is achieved by the domino C-acylation/O-acylation/aldolization sequence. This operationally simple one-pot elegant strategy provides structurally unique chromen-4-ones and isoflavones directly from phenols via concomitant formation of multiple C–C and C–O bonds in a single operation. The outcomes of the buttressing effect, substituent dependence, and catalyst and solvent specificity during the course of the Friedel–Crafts acylation reactions are demonstrated and supported by fitting experiments.

 Please wait while we load your content...
Please wait while we load your content...