Fischer indolisation of N-(α-ketoacyl)anthranilic acids into 2-(indol-2-carboxamido)benzoic acids and 2-indolyl-3,1-benzoxazin-4-ones and their NMR study†‡
Abstract
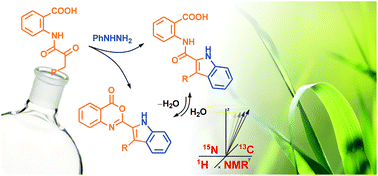
N-(α-ketoacyl)anthranilic acids reacted with phenylhydrazinium chloride in boiling acetic acid to afford 2-(indol-2-carboxamido)benzoic acids in good to excellent yields and 2-indolyl-3,1-benzoxazin-4-ones as by-products. The formation of the latter products could easily be suppressed by a hydrolytic workup. Alternatively, by increasing the reaction temperature and/or time, 2-indolyl-3,1-benzoxazin-4-ones can be obtained exclusively. Optimisations of the reaction conditions as well as the scope and the course of the transformations were investigated. The products were characterized by 1H, 13C and 15N NMR spectroscopy. The corresponding resonances were assigned on the basis of the standard 1D and gradient selected 2D NMR experiments (1H–1H gs-COSY, 1H–13C gs-HSQC, 1H–13C gs-HMBC) with 1H–15N gs-HMBC as a practical tool to determine 15N NMR chemical shifts at the natural abundance level of 15N isotope.

 Please wait while we load your content...
Please wait while we load your content...