Addition of optically pure H-phosphinate to ketones: selectivity, stereochemistry and mechanism†
Abstract
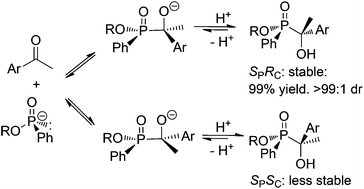
Aromatic methyl ketones and cyclic asymmetric ketones underwent hydrophosphorylation with P-stereogenic H–P species in the presence of potassium carbonate to produce P,C-stereogenic tertiary α-hydroxyl phosphinates in excellent yields with up to 99 : 1 dr. The diastereoselectivity was induced by a reversible conversion of less stable stereomer of product to that of a more stable one via an equilibrium, which was confirmed by aldehyde/ketone exchanging reaction. Toward the exchange, aliphatic or aldehyde carbonyl were more active than aromatic or ketone carbonyls, respectively. The stability difference between the two diastereomers was controlled by the sizes of substituents linking to phosphorus or α-carbon.

 Please wait while we load your content...
Please wait while we load your content...