A Lewis acid-mediated conformational switch†
Abstract
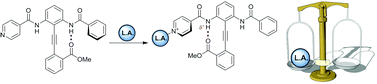
Molecules that change conformation in response to a stimulus have numerous uses, such as artificial chemoreceptors, novel drug delivery strategies and liquid crystal technology. Here we describe the design, synthesis and conformational behaviour of an isonicotinamide-substituted diphenylacetylene upon recognition of Lewis acids, including metalloporphyrins. Binding of these at a remote site – the pyridyl nitrogen – increases hydrogen-bond donor ability of the proximal amide NH, causing an increased preference for the alkyne rotamer in which this hydrogen bond is maintained.

 Please wait while we load your content...
Please wait while we load your content...