Switchable regioselectivity in the PIFA–BF3·Et2O mediated oxidative coupling of meso-brominated Ni(ii) porphyrin†
Abstract
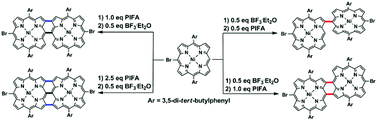
A simple and efficient method has been developed for the switchable synthesis of directly linked meso-brominated Ni(II) porphyrin dimers through PIFA–BF3·Et2O mediated oxidative coupling. The respective syntheses of meso–meso or meso–β singly, doubly, and triply linked porphyrin dimers can be easily realized with the same reagent system.

 Please wait while we load your content...
Please wait while we load your content...