Comparative perspective and synthetic applications of transition metal mediated oxidative cyclisation of 1,5-dienes towards cis-2,5-disubstituted tetrahydrofurans
Abstract
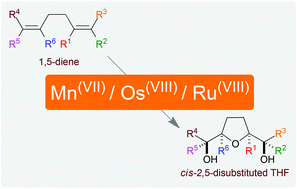
cis-2,5-Disubstituted tetrahydrofurans constitute the core of several natural products and synthetic analogues which exhibit a broad and interesting range of biological activities. This review highlights a personal perspective and provides a comparative note on the synthesis of cis-2,5-disubstituted tetrahydrofuran rings from 1,5-diene precursors using metal-oxo species. Also, mechanistic insights for these synthetically significant protocols are given and striking examples from the literature are reported, which draw attention towards the scope and synthetic utility of metal oxidants in the domain of cis-2,5-tetrahydrofuran containing bioactive natural product synthesis.

 Please wait while we load your content...
Please wait while we load your content...