Highly enantioselective phosphination and hydrophosphonylation of azomethine imines: using chiral squaramide as a hydrogen bonding organocatalyst†
Abstract
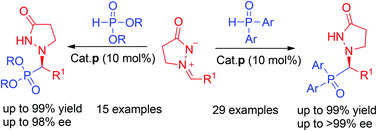
Enantioselective phosphination and hydrophosphonylation reactions between azomethine imines and diarylphosphine oxides or dialkyl phosphites were respectively developed by the use of a chiral squaramide as the hydrogen bonding organocatalyst, which afforded two types of phosphorus containing product in high yields with good to excellent enantioselectivities.
 Please wait while we load your content...
Please wait while we load your content...