Base-switched annuloselectivity in the reactions of ethyl malonyl chloride and imines†
Abstract
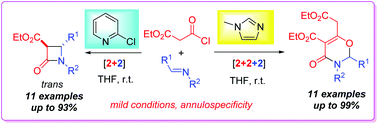
The base-switched annuloselectivity, namely [2 + 2] and [2 + 2 + 2] selectivity, in the reactions of ethyl malonyl chloride and imines is successfully realized. In the presence of the weak nucleophilic base 2-chloropyridine, the reactions deliver ethyl trans-β-lactam-3-carboxylates as the exclusive [2 + 2] products in up to 93% yields, while with the strong nucleophilic N-methylimidazole as the base, the reactions give rise to 2,3-dihydro-1,3-oxazin-4-one derivatives as the sole products in up to 99% yields via the formal [2 + 2 + 2] cycloaddition involving one molecule of the imine and two molecules of the ketene generated from malonyl chloride. Notably, ethyl trans-β-lactam-3-carboxylates are synthesized for the first time directly from the reactions of ethyl malonyl chloride and imines. Mechanistic discussions reveal that the annuloselectivity is controlled by the nucleophilicity of organic bases.

 Please wait while we load your content...
Please wait while we load your content...