Diversity-oriented synthesis of medicinally important 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) derivatives and higher analogs
Abstract
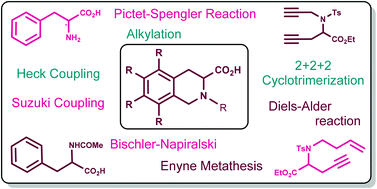
1,2,3,4-Tetrahydroisoquinoline-3-carboxylic acid (Tic) is a constrained analog of phenylalanine (Phe). The Tic unit has been identified as a core structural element present in several peptide-based drugs and forms an integral part of various biologically active compounds. This report covers the biological significance of the Tic core and provides a detailed account of various synthetic approaches available for the construction of Tic derivatives. Along with the traditional methods such as the Pictet–Spengler and Bischler–Nepieralski reactions, we cover various recent approaches such as enyne metathesis, [2 + 2 + 2] cycloaddition and the Diels–Alder reaction to generate Tic derivatives. In addition, syntheses of higher analogs of Tic are also discussed.

 Please wait while we load your content...
Please wait while we load your content...