Enantioselective copper catalysed C–H insertion reaction of 2-sulfonyl-2-diazoacetamides to form γ-lactams†
Abstract
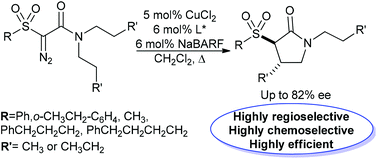
The first examples of asymmetric copper-catalysed intramolecular C–H insertion reactions of 2-sulfonyl-2-diazoacetamides are described; trans γ-lactams with up to 82% ee are achieved with the CuCl2-bisoxazoline-NaBARF catalyst system. The reactions generally display high efficiency and high trans selectivity, and also a strong regiochemical preference for insertion to lead to the formation of 5-membered rings over 4-membered rings. In cases where there are competing C–H insertion pathways available, to form sulfolanes or thiopyrans, only the insertion into the amide chain to form γ-lactams is observed. With phenylsulfonyl derivatives, a minor competing C–H insertion pathway leading to β-lactams is seen; interestingly, changing the identity of the copper ligand changes the product ratio of β/γ-lactams. The copper catalysed reactions compare favorably in terms of efficiency and enantioselectivity to the corresponding reactions catalysed by commercially available chiral rhodium catalysts.

 Please wait while we load your content...
Please wait while we load your content...