Adaptable synthesis of C-lactosyl glycoclusters and their binding properties with galectin-3†
Abstract
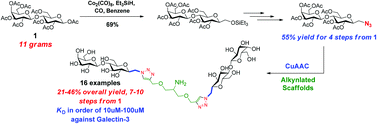
We report here the syntheses of mono- to tetravalent glycoclusters containing 1-methylene-C-β-lactose. The 1-methylene-C-β-lactose moiety has been synthesized from octa-acetyl-β-lactose using the key carbonyl insertion reaction and linked to a series of alkynlated scaffolds via CuAAC reaction to afford mono- to tetravalent glycoclusters. The binding affinities of the final products to galectin-3 were found in the range of 10–100 μM.

 Please wait while we load your content...
Please wait while we load your content...