Frustrated Lewis pair catalyzed hydrosilylation and hydrosilane mediated hydrogenation of fulvenes†
Abstract
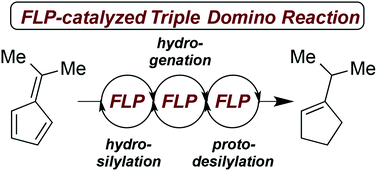
The frustrated Lewis pair (FLP) mediated hydrosilylation of pentafulvenes is described yielding allyl silanes with high regioselectivity in excellent yields. While phenyl substituted allyl silanes undergo B(C6F5)3-mediated rearrangement to vinyl silanes, dimethyl derivatives experience FLP-catalyzed hydrogenation followed by an unprecedented protodesilylation. This observation allowed the metal-free hydrogenation of 6,6-dimethylfulvene to iso-propyl cyclopentene according to a FLP-catalyzed triple domino reaction consisting of hydrosilylation, hydrogenation and protodesilylation. The mechanisms were investigated by deuteration experiments.

 Please wait while we load your content...
Please wait while we load your content...