Effect of conformational rigidity on the stereoselectivity of nucleophilic additions to five-membered ring bicyclic oxocarbenium ion intermediates†
Abstract
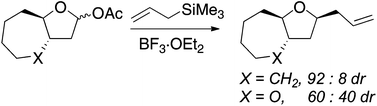
Nucleophilic substitution reactions of five-membered ring acetals bearing fused rings reveal that subtle changes in the structure of the fused ring can exert dramatic influences on selectivity. If the fused ring did not constrain the five-membered ring undergoing substitution, selectivity was comparable to what was observed for an unconstrained system (≥92% diastereoselectivity, favoring the product of inside attack on the oxocarbenium ion). If the ring were more constrained by including at least one oxygen atom in the ring, selectivity dropped considerably (to 60% diastereoselectivity in one case). Transition states of the nucleophilic addition of allyltrimethylsilane to selected oxocarbenium ions were calculated using DFT methods. These computational models reproduced the correlation between additional conformational rigidity and selectivity.

 Please wait while we load your content...
Please wait while we load your content...