Selective transamidation of 3-oxo-N-acyl homoserine lactones by hydrazine derivatives†
Abstract
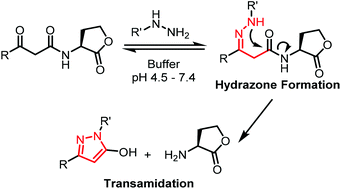
A method for the selective transamidation of the 3-oxo sub-family of N-acyl homoserine lactones (3-oxo-AHLs) under physiologically relevant conditions has been developed. The reaction has the potential to serve as a strategy for selective knockdown of key autoinducers in a multicellular environment.

 Please wait while we load your content...
Please wait while we load your content...