Abstract
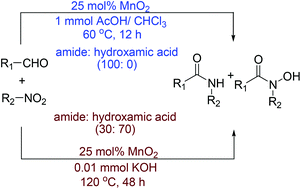
A new method for synthesis of amides and hydroxamic acids from nitroarenes and aldehydes is described. The MnO2 catalyzed thermal deoxygenation of nitrobenzene resulted in formation of a reactive nitroso intermediate which on reaction with aldehydes provided amides and hydroxamic acids. The thermal neat reaction in the presence of 0.01 mmol KOH predominantly led to formation of hydroxamic acid whereas reaction in the presence of 1 mmol acetic acid produced amides as the only product.

 Please wait while we load your content...
Please wait while we load your content...