One-pot synthesis of polysubstituted 3-acylpyrroles by cooperative catalysis†
Abstract
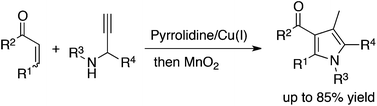
One-pot syntheses of polysubstituted 3-acylpyrroles from readily available unsaturated ketones and N-substituted propargylated amines have been developed. An aza-Michael/alkyne carbocyclization cascade, by cooperative catalysis using pyrrolidine and a copper salt, followed by oxidation in situ gave 3-acylpyrroles, which were also transformed further to functionalized, highly substituted 3-acylpyrroles.

 Please wait while we load your content...
Please wait while we load your content...