An efficient, Schiff-base derivative for selective fluorescence sensing of Zn2+ ions: quantum chemical calculation appended by real sample application and cell imaging study†
Abstract
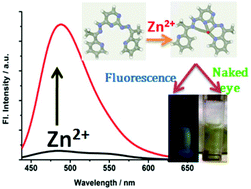
Since zinc ions (Zn2+) are involved in numerous biological phenomena and go through subsequent interactions with zinc-binding proteins, we have attempted a sensitive fluorescence based detection of this second most abundant metal ion using an engineered and synthesized Schiff-base ligand, namely 2,4-bis((Z)-2-(1-(pyridin-2-yl)ethylidene)hydrazinyl)pyrimidine (PyHP). The ligand exhibits a zinc-induced fluorescence response when investigated in a MeOH–buffer (10 mM HEPES, pH = 7) (4 : 1) solvent mixture. The presence of zinc ions (λex = 410 nm, quantum yield, ϕ = 0.20) causes approximately 45 fold fluorescence enhancement at 489 nm. Formation of the metal–ligand complex was ascertained by 1H NMR and mass spectra analysis. 1 : 1 binding affinity was ascertained according to Job's plot. Apart from this, theoretical interpretation of the experimental outcome was also obtained by applying density functional theory (DFT) to the PyHP-Zn2+ complex formation. The practical applicability of the ligand has been tested in bacterial cells as well as in mammalian cell imaging and also by measuring and comparing the amount of Zn2+ in some real samples such as liquid milk, tomato juice, banana stem juice and commercial fruit juice.

 Please wait while we load your content...
Please wait while we load your content...