Strategies for desymmetrising trehalose to synthesise trehalose glycolipids
Abstract
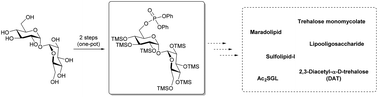
The desymmetrisation and regioselective protection of trehalose are major challenges in the chemical synthesis of biologically essential trehalose glycolipids. We reviewed the literature on desymmetrising trehalose to synthesise trehalose glycolipids and highlighted an efficient regioselective 6-O-phosphorylation method that can be applied to synthesise asymmetric trehalose glycolipids.

 Please wait while we load your content...
Please wait while we load your content...