Abstract
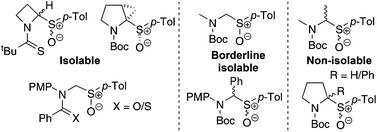
A synthetic study on the preparation of N-Boc α-amino sulfoxides has revealed an unexpected instability which is believed to be due to α-elimination of the sulfoxide to give an iminium ion. Full synthetic details are reported on two main synthetic routes: lithiation and sulfinate trapping of N-Boc heterocycles and oxidation of N-Boc α-amino sulfides. Six novel α-amino sulfoxides were successfully prepared and isolated. It is speculated that four other α-amino sulfoxides were synthesised but could not be isolated due to their propensity to α-eliminate the sulfoxide. Ultimately, a stable, cyclic N-Boc α-amino sulfoxide was prepared and this successful synthesis relied on the α-amino sulfoxide being part of a bicyclic [3.1.0] fused ring system that could not undergo α-elimination of the sulfoxide.

 Please wait while we load your content...
Please wait while we load your content...