A unifying mechanism for the rearrangement of vinyl allene oxide geometric isomers to cyclopentenones†
Abstract
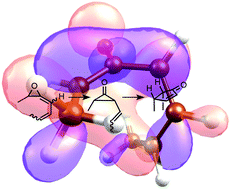
A new mechanism for the rearrangement of vinyl allene oxide geometric isomers to stereodefined cyclopentenones is proposed based on DFT computations. This mechanism comprises two steps, first the ring opening of the oxirane to give a vinylcyclopropanone, and then a [1,3]-C sigmatropic rearrangement. Depending primarily on the allene oxide double bond geometry the stepwise pathway is either competitive (for E allene oxides) or favored (for Z allene oxides) relative to the already described SN2-like concerted pathway. All bond-forming reactions take place through helically chiral transition states, which allows the stereochemical information of the substrates to be transferred to that of the products, in particular in the case of (enantiopure) Z allene oxides. In addition to revealing one more of the fascinating mechanisms with memory of chirality, the results deepen our understanding of the important jasmonate and clavulone biosynthetic pathways that occur in plants and corals.

 Please wait while we load your content...
Please wait while we load your content...