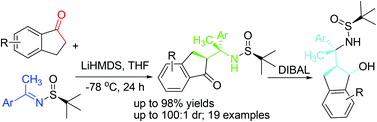
Highly efficient and generalized asymmetric synthesis of quaternary stereogenic carbon-containing β-amino indanones/indanoles via Mannich-type additions between 1-indanones and N-tert-butanesulfinylketimines†
Abstract
Here we report that, unlike other ketones, 1-indanone and acetophenone derived enolates undergo Mannich-type addition reactions with N-tert-butanesulfinyl ketimines with excellent yields (up to 98%) and diastereoselectivity (>99/1). The resulting compounds represent a new type of biologically relevant β-aminoketone derivative bearing quaternary stereogenic carbon, which could be further converted into the corresponding β-amino ketones and β-amino alcohols, possessing three consecutive stereogenic centres.
 Please wait while we load your content...
Please wait while we load your content...