Synthesis of 1-amino-2-aroyl/acetylnaphthalenes through a base mediated one pot inter and intramolecular C–C bond formation strategy†
Abstract
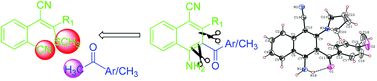
A new precursor 2-(1-cyano-2,2-bis(methylthio)vinyl)benzonitrile has been synthesized by the reaction of 2-cyanomethylbenzonitrile, carbon disulfide and methyl iodide under basic conditions. The reaction of 2-(1-cyano-2,2-bis(methylthio)vinyl)benzonitrile with various functionalized aryl/heteroaryl methyl ketones or acetone under basic conditions afforded 4-amino-3-aroyl/heteroaroyl/acetyl-2-methylsulfanylnaphthalene-1-carbonitriles in good yields through a (5C + 1C) annulation strategy; this involves sequential intermolecular, followed by intramolecular, C–C bond formation reactions. The structure of the product was confirmed by single crystal X-ray crystallography.
 Please wait while we load your content...
Please wait while we load your content...