Synthesis of novel 1,2,3-triazolyl derivatives of pregnane, androstane and d-homoandrostane. Tandem “click” reaction/Cu-catalyzed d-homo rearrangement†
Abstract
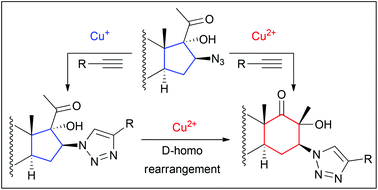
Copper-catalyzed 1,3-dipolar cycloaddition has been employed in the reaction of steroidal azides with various terminal alkynes. A number of novel 1,2,3-triazolyl derivatives of pregnane, androstane and D-homoandrostane were obtained in high yield (70–98%). The developed synthetic protocols allowed us to attach the triazolyl moiety to both the side chain and the steroidal backbone directly, despite the steric hindrance exerted by the polycyclic system. The presence of Cu(II) was shown to evoke D-homo rearrangement under mild conditions. A rational choice of the copper precatalyst permitted us to carry out the “click” reaction either along with tandem D-homo rearrangement or in the absence of this process. The tendency of 16-heterosubstituted steroids to undergo D-homo rearrangement under Cu(II) catalysis was studied.

 Please wait while we load your content...
Please wait while we load your content...