Catalytic, enantioselective vinylogous Michael reactions
Abstract
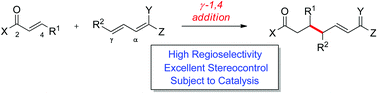
Recent progress in the field of catalytic, enantioselective vinylogous Michael reactions of latent dienolates is described which furnish optically highly enriched chiral 1,7-dioxo compounds of great utility in one synthetic operation. Emphasis is given to new catalysis modes which realise this challenging transformation with high regio- as well as enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...