Carbohydrate-based first stereoselective total synthesis of bioactive cytospolide P†
Abstract
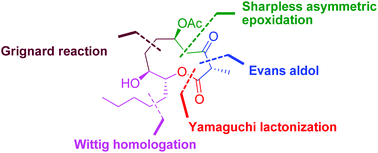
A facile carbohydrate-based highly stereoselective synthetic route has been developed for the cytospolide P (1) from D-ribose for the first time. Key steps of the synthesis include Wittig homologation, regioselective epoxide ring opening, Sharpless asymmetric epoxidation, Evans aldol reaction, and Yamaguchi macrolactonization.

 Please wait while we load your content...
Please wait while we load your content...