Total synthesis of an anticancer norsesquiterpene alkaloid isolated from the fungus Flammulina velutipes†
Abstract
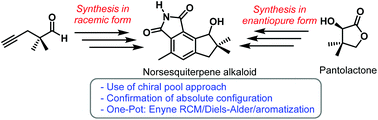
The first total synthesis of a norsesquiterpene alkaloid (R)-8-hydroxy-4,7,7-trimethyl-7,8-dihydrocyclopenta[e]isoindole-1,3(2H,6H)-dione, isolated from the mushroom-forming fungus Flammulina velutipes, in both racemic and enantiomeric pure forms, is reported. The (−)-enantiomer of the natural product has been synthesized from the D-(−)-pantolactone chiral pool. The synthesis features a one-pot, three-step reaction sequence comprising an enyne RCM/Diels–Alder/aromatization to construct the desired indane skeleton present in the natural product. Our synthesis further confirms the assigned structure and absolute configuration of the natural product.

 Please wait while we load your content...
Please wait while we load your content...