Helical folding of α/β-peptides containing β-amino acids with an eight-membered ring constraint†
Abstract
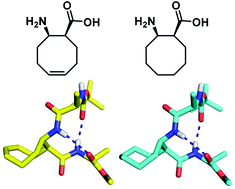
αβα-Tripeptide that contains a cyclic β-amino acid with an eight-membered ring, a cis-2-aminocyclooct-5-enecarboxylic acid (cis-ACOE) or a cis-2-aminocyclooctanecarboxylic acid (cis-ACOC) displayed an 11/9-helical turn in the crystal state. The related α/β-peptide oligomers were shown to adopt 11/9-helical conformations in solution.

 Please wait while we load your content...
Please wait while we load your content...