An enantioselective strategy for the total synthesis of (S)-tylophorine via catalytic asymmetric allylation and a one-pot DMAP-promoted isocyanate formation/Lewis acid catalyzed cyclization sequence†
Abstract
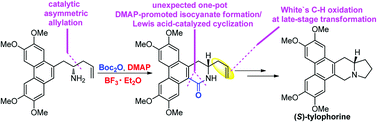
A new asymmetric total synthesis of a phenanthroindolizidine alkaloid (S)-tylophorine is reported, which features a catalytic asymmetric allylation of aldehydes and an unexpected one-pot DMAP promoted isocyanate formation and Lewis acid catalyzed intramolecular cyclization reaction. In addition, White's direct C–H oxidation catalyst system converting monosubstituted olefins to linear allylic acetates was also employed for late-stage transformation.

 Please wait while we load your content...
Please wait while we load your content...