Selective synthesis of (Z)-2-enynyl-2-hydroxy-imidazolidine-4,5-diones via Cu(i)-mediated multicomponent coupling of terminal alkynes, carbodiimides and oxalyl chloride†
Abstract
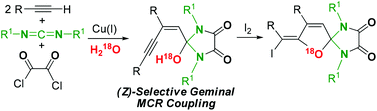
(Z)-2-Enynyl-2-hydroxy-imidazolidine-4,5-diones 2 are synthesized for the first time via Cu(I)-mediated (Z)-selective geminal coupling among two molecules of terminal alkynes, carbodiimides, and oxalyl chloride. Further transformation of 2a is performed to yield a highly functionalized spiro heterocyclic compound 5.

 Please wait while we load your content...
Please wait while we load your content...