Synthesis of N-alkyl isatins via oxidative cyclization of N-alkyl 2-bromo(chloro)acetanilides†
Abstract
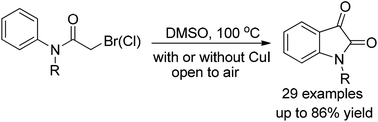
A highly efficient method for the synthesis of N-alkyl isatins starting from N-alkyl 2-bromo or 2-chloro acetanilides is described. The starting materials are easy to prepare and the yields of isatins are generally high. Operationally the reaction is very simple to run. Even though best results were obtained with a catalytic amount of CuI, the reactions of N-alkyl 2-bromo acetanilides actually performed well even in the absence of any metal catalyst.

 Please wait while we load your content...
Please wait while we load your content...