Hydroxyl radical induced oxidation of theophylline in water: a kinetic and mechanistic study†
Abstract
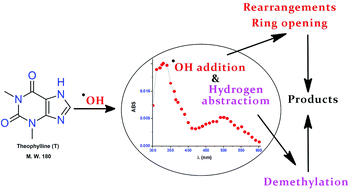
Oxidative destruction and mineralization of emerging organic pollutants by hydroxyl radicals (˙OH) is a well established area of research. The possibility of generating hazardous by-products in the case of ˙OH reaction demands extensive investigations on the degradation mechanism. A combination of pulse radiolysis and steady state photolysis (H2O2/UV photolysis) followed by high resolution mass spectrometric (HRMS) analysis have been employed to explicate the kinetic and mechanistic features of the destruction of theophylline, a model pharmaceutical compound and an identified pollutant, by ˙OH in the present study. The oxidative destruction of this molecule, for intermediate product studies, was initially achieved by H2O2/UV photolysis. The transient absorption spectrum corresponding to the reaction of ˙OH with theophylline at pH 6, primarily caused by the generation of (T8-OH)˙, was characterised by an absorption band at 330 nm (k2 = (8.22 ± 0.03) × 109 dm3 mol−1 s−1). A significantly different spectrum (λmax: 340 nm) was observed at highly alkaline pH (10.2) due to the deprotonation of this radical (pKa ∼ 10.0). Specific one electron oxidants such as sulphate radical anions (SO4˙−) and azide radicals (N3˙) produce the deprotonated form (T(−H)˙) of the radical cation (T˙+) of theophylline (pKa 3.1) with k2 values of (7.51 ± 0.04) × 109 dm3 mol−1 s−1 and (7.61 ± 0.02) × 109 dm3 mol−1 s−1 respectively. Conversely, oxide radicals (O˙−) react with theophylline via a hydrogen abstraction protocol with a rather slow k2 value of (1.95 ± 0.02) × 109 dm3 mol−1 s−1. The transient spectral studies were complemented by the end product profile acquired by HRMS analysis. Various transformation products of theophylline induced by ˙OH were identified by this technique which include derivatives of uric acids (i, iv & v) and xanthines (ii, iii & vi). Further breakdown of the early formed product due to ˙OH attack leads to ring opened compounds (ix–xiv). The kinetic and mechanistic data furnished in the present study serve as a basic frame work for the construction of ˙OH induced water treatment systems as well as to understand the biological implications of compounds of this kind.

 Please wait while we load your content...
Please wait while we load your content...