β-Amyrin synthase from Euphorbia tirucalli. Steric bulk, not the π-electrons of Phe, at position 474 has a key role in affording the correct folding of the substrate to complete the normal polycyclization cascade†
Abstract
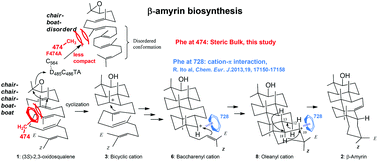
β-Amyrin, a triterpene, is widely distributed in plants and its glycosides confer important biological activities. Mutagenesis studies on β-amyrin synthase are very limited as compared with those of squalene-hopene cyclase and lanosterol synthase. This study was conducted to elucidate the function of the F474 residue of Euphorbia tirucalli β-amyrin cyclase, which is highly conserved in the superfamily of oxidosqualene cyclases. Nine site-specific variants with Gly, Ala, Val, Leu, Met, Tyr, Trp, His, and Thr were constructed. We isolated 9 products from these mutants in addition to β-amyrin and determined the chemical structures. The Gly and Ala mutants produced significantly larger amounts of the bicyclic products and a decreased amount of β-amyrin, indicating that the F474 residue was located near the B-ring formation site. Surprisingly, the Ala variant produced (9βH)-polypoda-7,13,17,21-tetraen-3β-ol and (9βH)-polypoda-8(26),13,17,21-tetraen-3β-ol, which are generated from a chair–boat folding conformation. This is the first report describing the conformational change from the chair–chair into the chair–boat folding conformation among the reported mutagenesis studies of oxidosqualene cyclases. Substitution with aliphatic amino acids lacking π-electrons such as Val, Leu, and Met led to a significantly decreased production of bicyclic compounds, and in turn exhibited a higher production of β-amyrin. Furthermore, the Leu and Met variants exhibited high enzymatic activities: ca. 74% for Leu and ca. 91% for Met variants as compared to the wild-type. These facts unambiguously demonstrate that the major role of Phe474 is not to stabilize the transient cation via cation–π interaction, but is to confer the appropriate steric bulk near the B-ring formation site, leading to the completion of the normal polycyclization pathway without accumulation of abortive cyclization products.

 Please wait while we load your content...
Please wait while we load your content...