Abstract
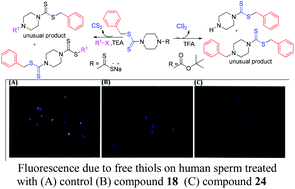
1-Substituted piperazinecarbodithioates were obtained by an unusual removal of CS2 in benzyl substituted dithiocarbamate derivatives under acid and basic conditions during design and synthesis of 1,4-(disubstituted)piperazinedicarbodithioates as double edged spermicides. A plausible mechanism for CS2 removal has been proposed. All synthesized compounds were subjected to spermicidal, antitrichomonal and antifungal activities. Twenty-one compounds irreversibly immobilized 100% sperm (MEC, 0.06–31.6 mM) while seven compounds exhibited multiple activities. Benzyl 4-(2-(piperidin-1-yl)ethyl) piperazine-1-(carbodithioate) (18) and 1-benzyl 4-(2-(piperidin-1-yl)ethyl)piperazine-1,4-bis(carbodithioate) (24) exhibited appreciable spermicidal (MEC, 0.07 and 0.06 mM), antifungal (MIC, 0.069–0.14 and >0.11 mM) and antitrichomonal (MIC, 1.38 and 0.14 mM) activities. The probable mode of action of these compounds seems to be through sulfhydryl binding which was confirmed by fluorescence labeling of sperm thiols.

 Please wait while we load your content...
Please wait while we load your content...