Palladium catalyzed N–H bond insertion and intramolecular cyclization cascade: the divergent synthesis of heterocyclics†
Abstract
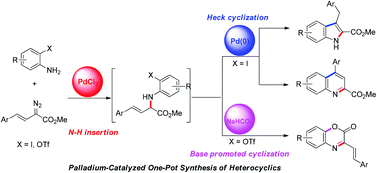
The palladium catalyzed carbenoid based N–H insertion and electronic effect controlled 5-exo-trig or 6-endo-trig mode Heck cyclization in one-pot has been realized for ortho-iodoanilines, in which PdCl2 was used as the single palladium resource. The corresponding indoles and quinolines were obtained respectively. However, for ortho-triflateanilines, base promoted cyclization is preferred and the lactones were obtained.

 Please wait while we load your content...
Please wait while we load your content...