A general synthetic strategy and the anti-proliferation properties on prostate cancer cell lines for natural phenylethanoid glycosides†
Abstract
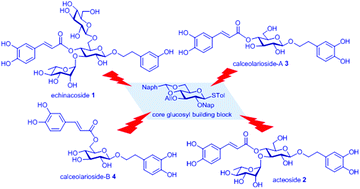
A general strategy for the synthesis of phenylethanoid glycosides (PhG) including echinacoside 1, acteoside 2, calceolarioside-A 3 and calceolarioside-B 4 is reported. The strategy features the application of low substrate concentration glycosylation and N-formyl morpholine modulated glycosylation methods for the construction of 1,2-trans β- and α-glycosidic bonds. The reported strategy does not invoke the use of the participatory acyl protecting function, which is incompatible with the ester function present in target PhG compounds. A preliminary study of the anti-proliferation properties of the PhG compounds 1–4 was performed; the acteoside 2 exhibited the best inhibition on the prostatic cancer cell proliferation.

 Please wait while we load your content...
Please wait while we load your content...