Protecting group free synthesis of urea-linked glycoconjugates: efficient synthesis of β-urea glycosides in aqueous solution†
Abstract
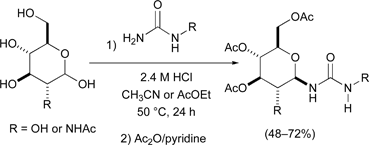
A method for the protecting group free synthesis of β-urea-linked glycoconjugates has been developed. The one step process, involving reactions between urea and D-glucose, N-acetyl-D-glucosamine or D-xylose in acidic aqueous solution, furnishes the corresponding β-urea glycosides in modest yields. This simple and efficient procedure is applicable to the synthesis of β-urea tethered amino acid–carbohydrate conjugates.

 Please wait while we load your content...
Please wait while we load your content...