Organocatalytic enantioselective hydrophosphonylation of aldehydes†
Abstract
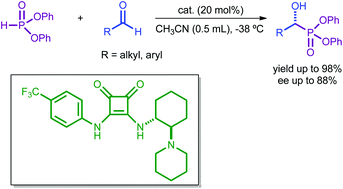
We report our results concerning the first squaramide-catalysed hydrophosphonylation of aldehydes. In all cases, the reactions proceeded smoothly and cleanly under mild reaction conditions rendering final α-hydroxy phosphonates in very good yields and high enantioselectivities. It is one of the few organocatalytic examples of this reaction using aldehydes. It is the first time that diphenylphosphite (1e) has been successfully employed in a chiral Pudovik reaction with aldehydes, in contrast to the dialkylphosphites used in previously published procedures, extending the generality of this asymmetric methodology.

 Please wait while we load your content...
Please wait while we load your content...