Synthesis of substituted 3-furanoates from MBH-acetates of acetylenic aldehydes via tandem isomerization–deacetylation–cycloisomerization: access to Elliott's alcohol†‡
Abstract
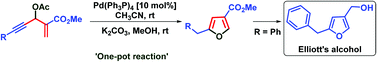
A new method for the synthesis of 5-substituted furan-3-carboxylates from Morita–Baylis–Hillman acetates of acetylenic aldehydes is reported. The process involves palladium-catalyzed isomerization followed by base-promoted deacetylation and cycloisomerization reactions. The utility of this chemistry is further demonstrated by the synthesis of Elliott's alcohol, a key intermediate of the pyrethroid resmethrins.

 Please wait while we load your content...
Please wait while we load your content...