Asymmetric synthesis of 2,5-disubstituted 3-hydroxypyrrolidines based on stereodivergent intramolecular iridium-catalyzed allylic aminations†
Abstract
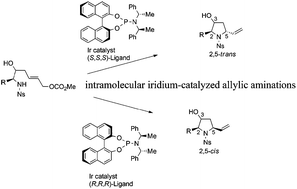
Intramolecular iridium-catalyzed allylic aminations of homochiral (E)-6-N-nosylaminohept-2-en-1-yl methyl carbonates were investigated. The relative position of the 2,5-substituents of the resulting pyrrolidines was found to be controlled by using both enantiomers (4 and 5) of the appropriate chiral ligand, demonstrating a simple and highly stereodivergent synthetic protocol. Selected trans- and cis-2,5-disubstituted 3-hydroxypyrrolidines (2a and 18a) were converted to (+)-bulgecinine (6) and (+)-preussin (7), respectively.

 Please wait while we load your content...
Please wait while we load your content...