Evolution of an oxidative dearomatization enabled total synthesis of vinigrol†
Abstract
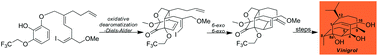
The evolution of the synthetic strategy resulting in a total synthesis of vinigrol is presented. Oxidative dearomatization/intramolecular Diels–Alder cycloaddition has served as the successful cornerstone for all of the approaches. Extensive radical cyclization efforts to form the tetracyclic core resulted in interesting and surprising reaction outcomes, none of which could be advanced to vinigrol. These cyclization obstacles were successfully overcome by using Heck instead of radical cyclizations. The total synthesis features a trifluoroethyl ether protecting group being used for the first time in organic synthesis. The logic of its selection and the group's importance beyond protecting the C8a hydroxyl group is presented along with a discussion of strategies for its removal. Because of the compact tetracyclic cage the route is built around many unusual reaction observations and solutions have emerged. For example, a first of its kind Grob fragmentation reaction featuring a trifluoroethyl leaving group has been uncovered, interesting interrupted selenium dioxide allylic oxidations have been observed as well as intriguing catalyst and counterion dependent directed hydrogenations.

 Please wait while we load your content...
Please wait while we load your content...